-40%
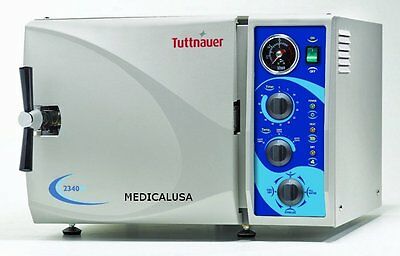
18L Dental Autoclave Steam Sterilizer Medical Sterilizition With Drying Function
$ 528
- Description
- Size Guide
Description
Ship from USA to USA.Voltage:110V / 50Hz
Heater Power: 1100W
Tank capacity: 18L
Inside dimensions: Φ250 x D350mm
Sterilize temperature: 122℃ & 134℃
Sterilize pressure: 0.12Mpa & 0.23Mpa
Sterilize time: 25min & 6min
Drying time: 40min
Tank Material: Stainless steel #304
Machine size (L*W*H): 60 * 39 * 36cm
Packing size (L*W*H): 61 * 53 * 40cm
N.W.: 33KG
G.W.: 35KG
Standard accessories: (Plate * 3PCS; Shelf * 1PCS; Plate Taking tool * 1PC)
Application: For dental, surgery, beauty salon, medical care, laboratory, and so on.
Caution:
1. After the completion of disinfection,please to wait for 10 minutes.
Pressure Gauge in red area. Do not rotate handle, open the steam discharge valve firstly.
2. Before each use,must supply water 800ml of distilled water .
3. Please close steam discharge valve before the disinfection.
4. Do not use any corrosive or chemical water as the disinfection.
5. Do not share the same electricoutllet with other electrical apparetus.
6. Please pay attention to correct voltage and frequency.
7. Connect steam exhaust pipe with the steam vent,before operation.
FDA Statement
The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local
regulatory agencies. If so, you can bid on this item only if you are an authorized purchaser. If the item is
subject to FDA regulation, I will verify your status as an authorized purchaser of this item before shipping of
the item.
Seller name:dr.baistra
City,State:SHENZHEN,China
Telephone number:0755-32870423
Re: K181087
Trade/Device Name: Apex Locator, DPEX III
Regulatory Class: Unclassified
Product Code: LQY
Dated: September 8, 2018
Received: September 19, 2018
K181993
Device Classification Name Sterilizer, Steam
510(K) Number K181993
Device Name STURDY Autoclave Super Microm (Models SA-260MA And SA-260MA-R)
Regulation Number 880.688
Classification Product Code FLE
Date Received 7/26/2018
Decision Date 11/1/2019
Device Class 2
Regulation Number 21CFR880.6880